Description
Physarum polycephalum slime mold culturing kit
Choose one of the 2 options-
Either 2pc Physarum polycephalum or the whole kit
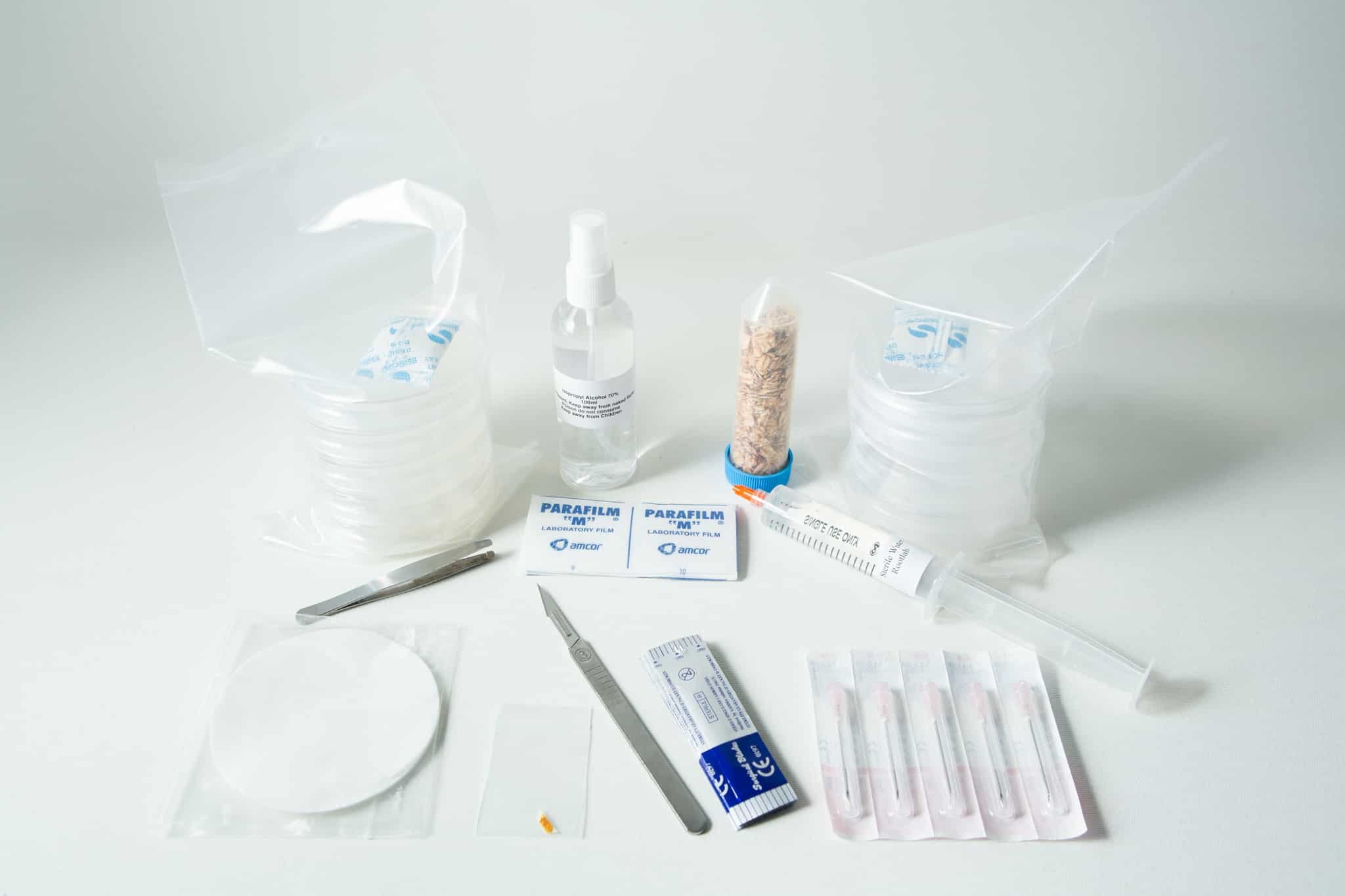
What is included in your kit: (Use kit within 45 days of receipt)
1)2 pc Screlotum of Physarum polycephalum (Always store in the dark)
2)70% isopropyl alcohol 100ml
3)Sterilised oats always keep lid tightly secured, store at room temperature.
4)5pcs-2% non-nutrient water agar plate
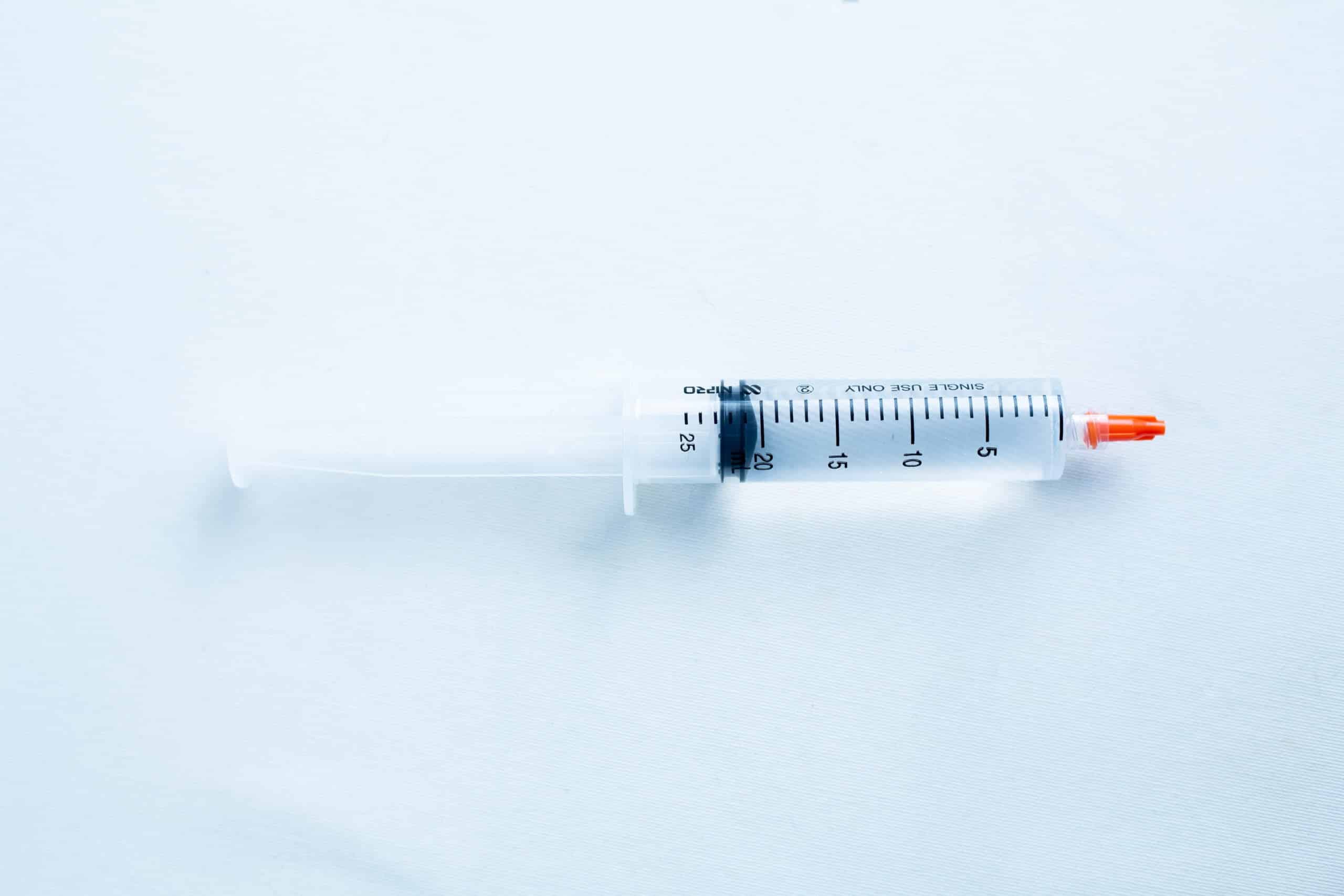
5)1 X20ml sterile water syringe
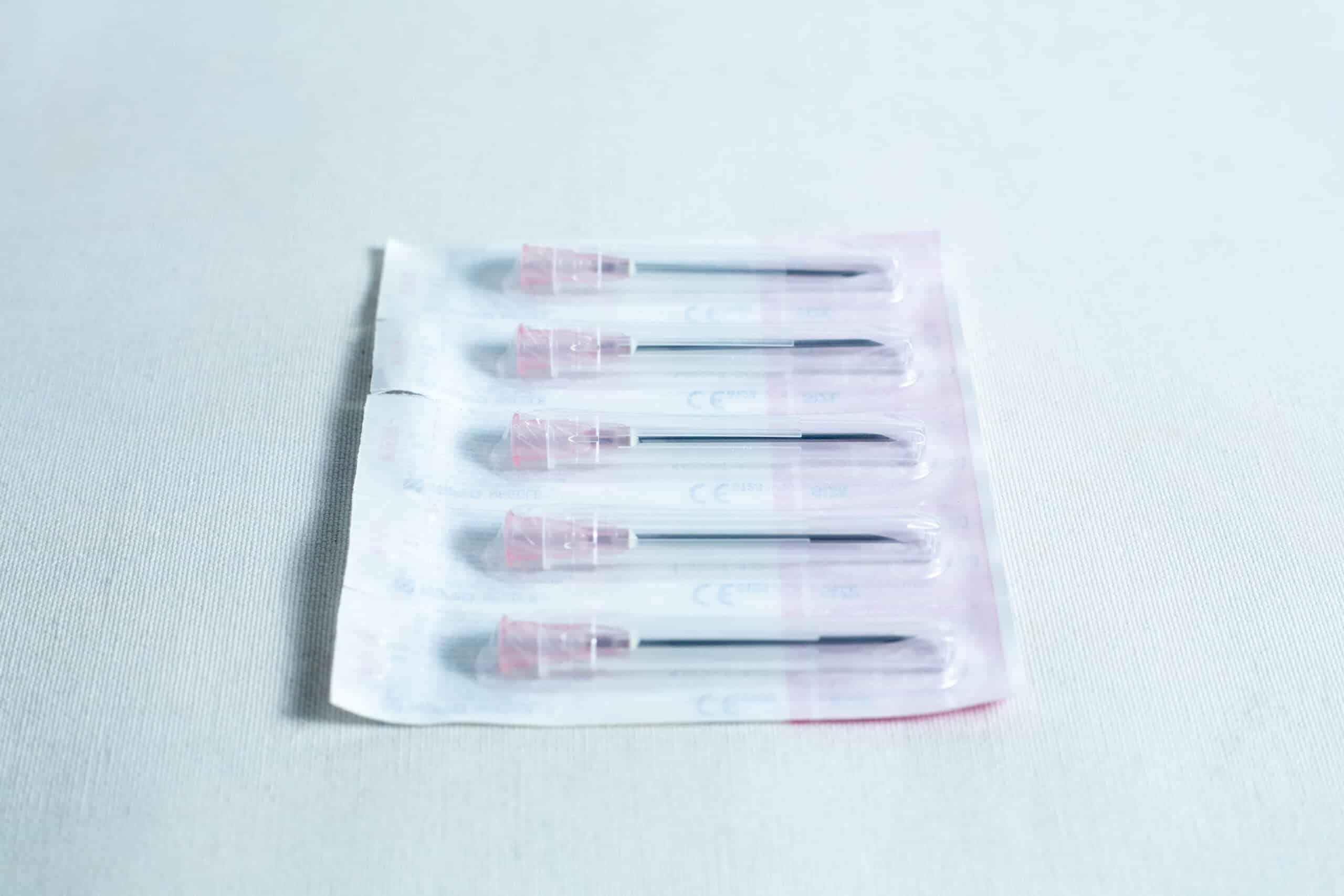
6)5 needles (single use)
7)5 sterile empty plates
8)Scalpel and 10 sterile blades
9)5 Sterile Paper filter
10)10 pre cut parafilm
11)Tweezers
To avoid contamination, all procedures must be conducted using sterile techniques in a still environment. Please use 70% isopropyl alcohol to sanitise all your work surfaces and tools before starting. Use a complete still room without a fan, air conditioning, or the door or window open.
Familiarise yourself with the conditions the Department of Agriculture, Fisheries, and Forestry imposes on you. If you purchase this product, you agree to abide by these conditions.
The goods must not be exposed to or used on animals other than laboratory animals and must not be used in plants, humans, or the environment. Laboratory organisms are guinea pigs, hamsters, mice, rats, rabbits, or microorganisms under laboratory or animal house conditions.
Rootlab recommends not removing any label from the product and ensuring everyone in the team knows about these conditions.
Before you purchase this kit, remember that it’s not for beginners; proper sterile technique and disposable knowledge are needed. The kit should be used by adults over 18+ only and kids under the supervision of an adult. Improper technique will lead to contamination or no growth. We do not accept a refund or exchange of this kit.
A) Culturing *Physarum* from Sclerotia
-
Dispense a single drop of sterile water from the syringe directly onto the center of a 2% agar plate. You may also use one of the provided needles for better control of the water flow—this helps avoid adding excess water. Do not use more than one drop. Each time you remove the sterile water syringe cap, make sure to place it on a clean surface. After using the syringe, spray the cap with isopropyl alcohol before replacing it. Store the syringe either at room temperature in a zip-lock bag or in the refrigerator. Any unused agar plates should be stored in a cupboard at room temperature.
-
Using the included tweezers, place one sclerotium face down onto the water droplet. The kit includes two sclerotia. Before use, sterilise the tweezers either by heating or by spraying with isopropyl alcohol and allowing them to sit for at least 20 seconds.
-
Take approximately 10 oatmeal flakes from the oat container and position them around the sclerotium. You can gently shake the flakes out of the container. Be sure to close the lid securely afterward to maintain sterility of the oats.
-
Cover the agar plate with its lid and seal it using parafilm. Place the plate in a dark area with the lid facing up—such as a cupboard or drawer—and wait 8 to 16 hours for the sclerotium to develop into a plasmodium.
-
After formation, the plasmodium will begin to move from the filter paper onto the oat flakes and across the agar surface.
-
Continue to feed the plasmodium by adding more oat flakes as needed. If left without food, it may attempt to escape the petri dish. Under dry conditions or if food is insufficient, it may produce spores or revert to its sclerotium state.
-
Once the plasmodium has fully spread across the original agar plate, you can transfer (subculture) it onto a fresh plate.
B) Subculturing *Physarum* (from an Active Culture)
To transfer *Physarum* to a fresh plate:
1. Prepare (heat-sanitise) a scalpel for cutting and transferring agar sections.
2. Use the sterilized scalpel to cut out a 1-cm square of agar containing plasmodium from an active culture plate.
3. Transfer this agar square onto a fresh 2% agar plate with its plasmodium side facing downward.
4. Add approximately 10 oat flakes to feed the plasmodium. Wrap the plate with Parafilm.
C) Inducing *Physarum* to Form Sclerotia
To encourage *Physarum* to form sclerotia:
1. Place a small piece or 1/4-cut sterile filter paper on a new 2% agar plate and moisten it with sterile water before lightly sprinkling sterile oat flakes over it.
2. Using previously described subculturing methods, transfer a 1-cm square of agar containing plasmodium onto this filter paper with its plasmodium side facing downward.
3. Seal the plate with parafilm and allow the plasmodium to grow until it completely covers the filter paper.
4. During this growth phase, Prepare a larger sterile filter paper inside an empty agar plate.
5. Once growth on the smaller filter paper is complete, moisten the larger piece with sterile water inside its container.
6. Use sterilized tweezers to transfer the smaller plasmodium-covered filter paper (along with any oat flakes) onto the center of the larger filter paper.
7. Allow time for the plasmodium to spread across most of the larger filter paper before removing the smaller piece containing oat flakes to starve it.
8. Gradually dry out the larger filter paper by partially opening its container’s lid; as it dries, sclerotium will form.
D) Sporulation
Facilitating sporulation can be challenging, as it is typically carried out under tightly regulated conditions in research laboratories. However, sporulation can occur more reliably if the following steps are adhered to:
1. Allow the plasmodium to cover the plate and deplete its food resources completely.
2. Position the plate upside down in a location exposed to daylight, but avoid placing it directly near a window.
3. Be patient. Sporulation might take several days or even longer. It is unlikely to happen if it does not occur within two weeks.
Slime molds are imported into Australia under a permit, and the kit price includes the permit’s application and maintenance cost. Video coming soon!